Fibroblast growth factor (FGF) constitutes a large family of polypeptide growth factors found in a variety of multicellular organisms, including invertebrates.
The function of FGFs is not restricted to cell growth. Instead, FGFs are involved in diverse cellular processes including chemotaxis, cell migration, differentiation, cell survival, and apoptosis. The common feature of FGFs is that they are structurally related and generally signal through receptor tyrosine kinases. FGFs play an important role in embryonic development in invertebrates and vertebrates.
The human FGF protein family consists of 22 members that share a high affinity for heparin as well as a high-sequence homology within a central core domain of 120 amino acids, which interacts with the FGFR.
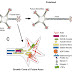
Structure of a generic FGF protein contains a signal sequence and the conserved core region that contains receptor- and HSPG-binding sites. The main structural features of FGFRs including Ig domains, acidic box, heparin-binding domain, CAM-homology domain (CHD), transmembrane domain, and a split tyrosine kinase domain are illustrated with respective functions: CAM, Cell adhesion molecule; ECM, extracellular matrix; PKC, protein kinase C.
FGFR signal transduction
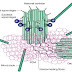
The signal transduction starts soon after the FGF binds to the Ig domain III. Binding of FGFs causes receptor dimerization and triggers tyrosine kinase activation leading to autophosphorylation of the intracellular domain. Tyrosine autophosphorylation controls the protein tyrosine kinase activity of the receptor but also serves as a mechanism for assembly and recruitment of signaling complexes. These phosphorylated tyrosines function as binding sites for Src homology 2 and phosphotyrosine binding domains of signaling proteins, resulting in their phosphorylation and activation. A subset of Src homology 2-containing proteins such as Src-kinase and phospholipase Cγ (PLCγ) possesses intrinsic catalytic activities, whereas others are adapter proteins. FGF signal transduction, as analyzed during early embryonic development, can proceed via three main pathways and i.e.
Ras/MAPK pathway
The most common pathway employed by FGFs is the MAPK pathway. This involves the lipid-anchored docking protein FRS2 (also called SNT1) that constitutively binds FGFR1 even without receptor activation. Several groups have demonstrated the importance of FRS2 in FGFR1-mediated signal transduction during embryonic development. After activation of the FGFR, tyrosine phosphorylated FRS2 functions as a site for coordinated assembly of a multiprotein complex activating and controlling the Ras-MAPK signaling cascade and the Phosphatidylinositol 3 (PI3)-kinase/Akt pathway. The FRS2 tyrosine phosphorylation sites are recognized and bound by the adapter protein Grb2 and the protein tyrosine phosphatase (PTP) Shp2. Grb2 forms a complex with the guanine nucleotide exchange factor Son of sevenless (SOS) via its SH3 domain. Translocation of this complex to the plasma membrane by binding to phosphorylated FRS2 allows SOS to activate Ras by GTP exchange due to its close proximity to membrane-bound Ras. Once in the active GTP-bound state, Ras interacts with several effector proteins, including Raf leading to the activation of the MAPK signaling cascade. This cascade leads to phosphorylation of target transcription factors, such as c-myc, AP1, and members of the Ets family of transcription factors.
PLCγ/Ca2+pathway
The PLCγ/Ca2+ pathway involves binding of PLCγ to phosphorylated tyrosine 766 of FGFR1.Upon activation, PLCγ hydrolyzes phosphatidylinositol-4,5-diphosphate to form two second messengers, inositol-1,4,5-triphosphate and diacylglycerol. Diacylglycerol is an activator of protein kinase C, whereas inositol-1, 4, 5-triphosphate stimulates the release of intracellular Ca2+. This cascade has been implicated in the FGF2-stimulated neurite outgrowth (48, 49) and in the caudalization of neural tissue by FGFR4 in Xenopus.
PI3 kinase/Akt pathway
The PI3 kinase/Akt pathway can be activated by three mechanisms after FGFR activation, and the phospholipids thereby generated regulate directly or indirectly the activity of target proteins such as Akt/PKB.
Among other processes, the PI3 kinase signaling branch is involved during Xenopus mesoderm induction acting in parallel to the Ras/MAPK pathway. Overexpression of a dominant negative form of the PI3 kinase-regulatory subunit p85 interferes with Xenopus mesoderm formation. Conversely, co expression of activated forms of MAPK and PI3 kinase leads to synergistic mesoderm induction.
















1 comments:
Fibroblast Growth Factor-Basic is a growth factor encoded by the FGF2 gene. It is found in basement membranes and sub-endothelial extracellular matrix. FGF2 specifically binds to fibroblast growth factor receptor (FGFR) proteins. Fibroblast Growth Factor-Basic
Post a Comment